Effects of barley extract on the growth of algae Spirogyra, Synedra, and Ankistrodesmus
Effects of barley extract on the growth of algae Spirogyra, Synedra, and Ankistrodesmus
Brooke Burmeister
Environmental Science Program, Southwest Minnesota State University
Abstract
Excessive amounts of algae are a nuisance in lakes. Experiments were conducted to determine the efficacy of barley straw extract in controlling algal growth in three freshwater algae. Spirogyra (filamentous green algae) , Synedra (diatom) , and Ankistrodesmus (single celled green algae) were exposed to barley straw extract in an environmental chamber for nine days. Algae was exposed in 50 mL test tubes with five replicates of each treatment, five replicates of algae with no barley extract (control) and five tubes barley with no algae. Chlorophyll concentrations were analyzed at the beginning and end of the study to determine effects on the growth of the algae. All three species of algae grew in the unexposed controls, but growth was statistically significantly reduced for all three species when exposed to barley extract (ANOVA, p= 0.05). Results indicate that barley could be used as an algistatic agent to control algae growth.
Introduction
An excessive amount of algae can be a nuisance in lakes. Large blooms of blue-green algae cause toxicity to fish (Everall and Lees 1996). Some algae have noxious odors and a bad taste that livestock will not drink and humans will not use. Phytoplankton can also be toxic and cause fish kills. The general public also reduce their use of lakes for fishing and swimming (Ferrier et al. 2005). Reducing the amount of noxious algae will benefit the fish and the visitors to the lake. In the past, additions of chemicals to the lake have reduced the amount of algae present but there may be a more eco-friendly alternative; research has shown that barely straw could be this answer (Ball et al. 2000).
Studies have shown that decomposing barley straw is growth inhibiting to algae. Lab research using chopped barley straw reduced the growth of harmful cyanobacteria, Microcystis aeruginosa . The algae cells were not killed but the cell densities began to decline after the first day. Barley straw could be used as an algistatic agent rather than an algaecide (Xi et al. 2010). A field study in England recommended often using netted barley straw will reduce the growth of algae and improve water clarity. Barley straw does not eliminate the algae present, instead it prevents growth of new algae (Lembi 2002).
Research using barley straw extract created using decomposing barley straw has had similar results. In laboratory experiments barley straw extract was applied to Prymnesium parvum for a four week period. Both high and low doses of barley straw extract lowered the amount of algae growth (Grover et al. 2007). Microcystis aeruginosa growth was also inhibited by using a barley straw extract (Ball et al. 2000).
Synedra is a diatom that has long needle shaped cells. Diatoms are small phytoplankton that live in a shell made of silica which sinks to the bottom when the organism dies. The silica plays a role in global cycling through the aquatic food web. The shells are currently used in pool filters, kitty litter, and water treatment systems (Hosja, 1998). Graham et al. (2009) states that diatoms are commonly found in oceans, lakes, slow flowing rivers, and streams. When diatoms become too thick they can cause harmful growth by developing an odor in drinking water and block water treatment filters (Bellinger and Sige 2010).
Ankistrodesmus is another type of green algae but is single celled. It is found in all types of freshwater, artificial ponds (Graham et al. 2009) eutrophic lakes, and slow flowing rivers. Ankistrodesmus occurs in bundles and groups in more acidic waters (Bellinger and Sigee 2010). There has not been much research done on the ecology of Ankistrodesmus.
Materials and Methods
Algae Exposures
Spirogyra, Synedra, and Ankistrodesmus algae cultures were purchased from Carolina Biological Company. A total of 35 sterile 50 mL screw cap test tubes were filled with 9mL of sterile Alga-Gro nutrient (Carolina Biological Company). Five replicates for each algae species also received 7mL of algae from the algae cultures and 9mL of barley extract (Pondlife Co.). Another five replicates of positive control test tubes for each algae species contained 7mL of algae and 9mL of pasteurized spring water. Five negative control replicate test tubes contained 7mL of pasteurized spring water and 9mL of sterile barley extract. All test tubes caps were placed on loosely to allow gases to be exchanged. Test tubes were placed in the Percival PGC-10 environmental chamber for nine days with a temperature of 21°C and a 12 hour photoperiod with 157 Lux. To promote gas exchange in the test tubes and to keep algae suspended, the test tubes were agitated every 48 hours.
Assessment of Growth and Analysis
To determine the effects of the barley extract on the growth of the algae, chlorophyll concentrations were measured at the beginning of the study and after nine days of exposure. Each test tube was glass-fiber filtered (Gelman AE; effective pore size 0.7µm) and dried using four drops of MgCO₃ to stabilize the samples for chlorophyll analysis (Parsons et al. 1984). The initial samples were then frozen until the end of the experiment when all samples were analyzed. A tissue grinder was used to disrupt the cells. Samples were placed into 50mL test tubes with screw caps and 15mL of 90% acetone was added to each test tube and shaken. Samples were placed into a dark refrigerator at 4°C (APHA 1989). After two hours test tubes were placed in the Beckman J-58 centrifuge for 15 minutes at 500rpm. The liquid was decanted into a 1 cm spectrometer cuvette and absorbance read at wavelengths of 750 and 650 nm on a Spectronic 20D spectrometer. Corrections for phaeo-pigments and particulates were made by adding 0.1 mL of hydrochloric acid (HCL) to each sample. Samples then were read on the spectrophotometer (Spectronic, 20D) at 750nm and 664nm (APHA 1989).
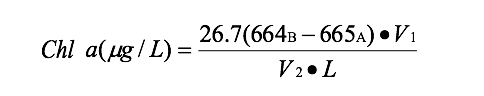
V 1=Volume of extract 664 B =664-750nm before acid
V 2=Volume of filtered 665 A =665-750nm after acid
L=Path of length (1cm)
Results
Three species of algae Spirogyra, Synedra, and Ankistrodesmus were grown in the lab with and without barley extract. Readings of chlorophyll were taken initially and at the end of a nine day exposure with barley extract and without.
The means and standard errors (SE) were calculated and an ANOVA was used to determine if the difference in algae growth (i.e. chlorophyll concentration) was statistically different between the treatment group (with barley) and the positive control. There was statistical difference between the two groups.
Evidence showed that Spirogyra with no barley extract (untreated group) grew significantly during the nine day period in the environmental chamber. Synedra and Ankistrodesmus initial growth was also higher than the untreated group. All three algae species exposed to barley extract had lower levels of chlorophyll when compared to the untreated groups after nine days of exposure.
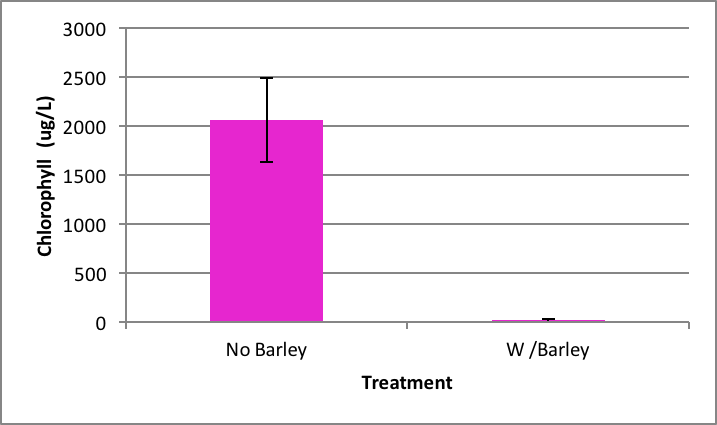
The growth of Spirogyra was significantly inhibited by barley extract (Figure 1). After the nine day period chlorophyll was hardly detectable at an average of 20.50 µg/L ±SE 16.13 in barley straw treatment compared to the control that had an average chlorophyll content of about 2060.81 µg/L ±SE 426.34 (Figure 1). Results indicated that the barley extract did statistically significantly reduce the growth of Spirogyra (p=0.000) compared to the positive control.
|
Figure 1. Average concentration of chlorophyll in Spirogyra after nine days exposure to barley ± SE (n=5); *statistically significant difference. |
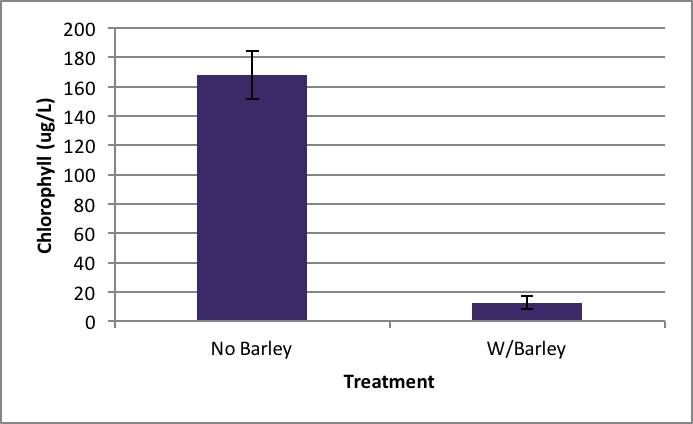
Ankistrodesmus growth was also statistically inhibited by barley extract. After the nine day experiment the positive control (no barley extract) had an average of 167.89 µg/L ±SE 16.64 of chlorophyll present (Figure 2). The treatment group (with barley extract) had a detection of chlorophyll at a significantly lower rate of 12.82 µg/L ±SE 4.53. ANOVA was used to test the probability of this result happening at random and it was very low at p= 0.007.
|
Figure 2. Average concentration of chlorophyll in Ankistrodesmus after nine days exposure to barley ± SE (n=5); *statistically significant difference. |
There was also significant inhibition of Synedra growth when it was exposed to barley extract compared to no barley extract present. The probability of this occurring is 0.000 according to the ANOVA. Synedra with barley had a chlorophyll concentration of 15.38 µg/L ±SE 4.35 compared to without barley which had 55.11 µg/L ±SE 14.84 (Figure 3).
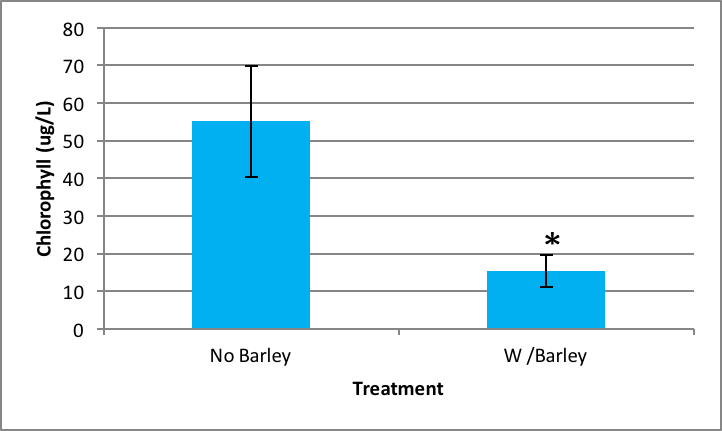
|
Figure 3. Average concentration of chlorophyll in Synedra after nine days exposure to barley ± SE (n=5); *statistically significant difference. |
An evaluation was also done of the chlorophyll concentration in the test tubes at the beginning of the experiment (“initial”) and after nine days of growth in control tubes (no barley extract) to determine if the three algae samples were growing over the course of the experiment.
Shown in Figure 4 after nine days in the environmental chamber the Spirogyra controls (without barley extract) grew significantly. The initial chlorophyll concentration was 359.95 µg/L ±SE 56.24 compared to the end concentration of 2060.81 µg/L ±SE 426.34 µg/L (Figure 4). Ankistrodesmus chlorophyll concentration at the beginning of the experiment (273.94 µg/L ±SE 90.98) was higher than the ending control chlorophyll concentrations (167.89 µg/L ±SE 16.64), although not statistically significant different (Figure 5). An evaluation of the Synedra chlorophyll concentrations at the beginning of the nine day experiment showed a significant reduction in growth of the control algae from 347.35 µg/L ±SE 9.54 to 55.11 µg/L ±SE 14.84 chlorophyll concentration (Figure 6) (p < 0.05).
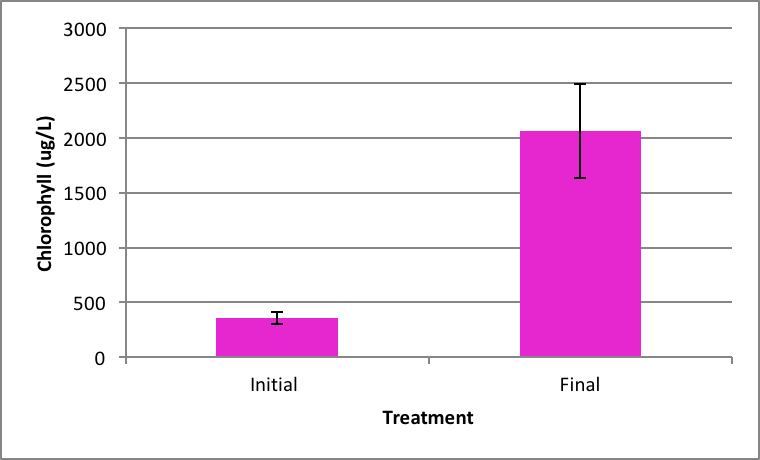
|
Figure 4. Average concentration of chlorophyll in Spirogyra at the beginning of the (initial) ± SE and after the nine day experiment without barley ± SE. (n=5); *statistically significant difference. |
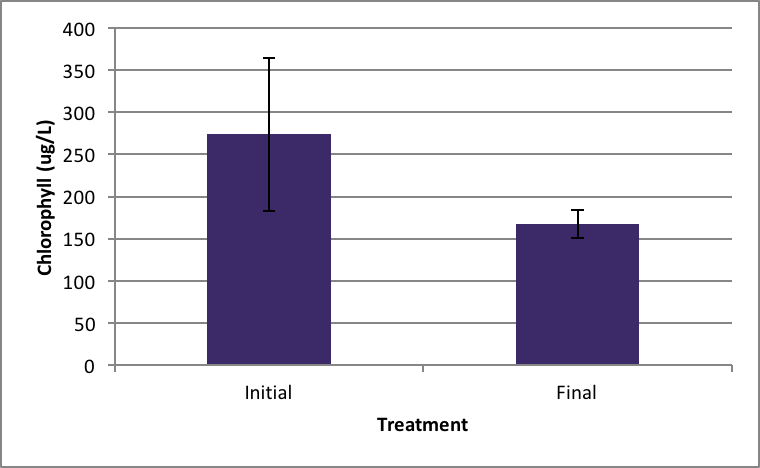
|
Figure 5. Average concentration of chlorophyll in Ankistrodesmus at the beginning of the experiment (initial) ± SE and after the nine day experiment without barley ± SE. (n=5). |
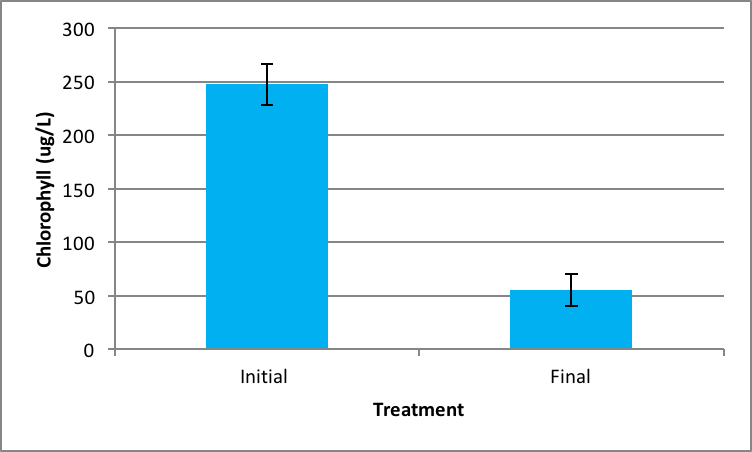
|
Figure 6. Average concentration of chlorophyll in Synedra at the beginning of the experiment (initial) ± SE and after the nine day experiment without barley ± SE. (n=5); *statistically significant difference. |
The appearance of the algae exposed to barley extract changed over the nine day experiment compared to the positive control. By the second day the Spirogyra sank to the bottom of the treatment test tubes compared to the positive control which were at the top of the test tubes. Filaments were also shorter in length compared controls. Ankistrodesmus turned brown and Synedra changed to a white color.
By day six, Synedra treatment group was almost clear with a faint cloudy appearance; Spirogyra went from the green color to a white cotton and were still at the bottom of the test tubes Ankistrodesmus had a white film on the bottom of the test tubes.
Discussion
Barley straw extract was effective at inhibiting the growth of algae in all three species Spirogyra, Ankistrodesmus, and Synedra . The methods followed were similar to previous research performed by Ferrier and others (Ferrier et al. 2005) except in this project purchased barley extract (PondLife Co.) was used. Ferrier et al. (2005) prepared barley extract from decomposing barley and then tested unfiltered and sterile filtered extract on 12 species of algae, including Spirogyra and Synedra.
Ferrier et al. (2005) found that Spirogyra growth was significantly reduced compared to the control when exposed to sterile filtered extract. These results are similar to effects seen with this project Spirogyra growth was significantly reduced with exposure to barley extract. Results compared on the algae Synedra are, however, different. Their results (Ferrier et al. 2005) showed no significance difference in growth between the control, unfiltered, and sterile filtered barley straw extract. Results of the current study did show a statistically significant (p<0.05) reduction in growth compared to the control. The difference in response of Synedra between the two studies may be due to the differences in barley extract source. This project purchased commercial barley extract and Ferrier et al. (2005) produced their own extract in the lab.
A study conducted by Geiger et al. (2005) on Ankistrodesmus exposed to decomposing barley straw showed that Ankistrodesmus growth was suppressed, which supports the results found with the current study.
Another study conducted in the United Kingdom used lab prepared barley straw extract and collected lake water samples to obtain the algae samples. They had similar results using lake water they collected and tested in a lab. The lake water contained the algae species Scenedesmus, Microcystis, Chlorella, and Anabaena. In the presence of barley straw extract there was no algae growth of these four species recorded during a 28 day experiment (Ball et al. 2000).
Spirogyra controls showed good growth over the course of the study. Ankistrodesmus had a slight drop in growth in the controls, although not significantly different from the starting chlorophyll concentration. Synedra, however, had a large drop in chlorophyll concentration in the controls from the beginning to the end of the experiment. Reasons for Synedra decreasing in growth in the positive control (no barley extract) could be related to the fact that diatoms need extra nutrients in order to grow. According to James (1978) diatoms can grow on a variety of different media but they need a silicate source to have good growth. It is recommended that 10 mg/L of sodium metasilcate be added to media to produce good growth. The growth media used in this study was a consistent formulation for all test containers and did not include increased silica content.
Another difference in the response of the three algae species tested in this study is the order of magnitude in response. All three species of algae had initial chlorophyll concentrations of approximately 250-350 µg/L. Over the course of the experiment, Spirogyra chlorophyll concentration in the controls increased to over 2000 µg/L. Ankistrodesmus and Synedra ending chlorophyll concentrations in the controls were both under 200 µg/L.
The inhibiting effects of barley extract might result from the chemical compounds hydrogen peroxide (Zhou 2010) and oxidized phenolics (Everall and Lees 1996; Ball et al. 2000; Zhou 2010). These compounds occur during the decomposing process. Zhou (2010) analyzed chemical compounds in decomposing barley straw. It was found that butylated hydroxytouluene and 2-methoxy-4-vinylphenol occurred in many of the samples they observed. These chemical compounds qualify as candidates for further research on effects of algal growth.
Results of this research project and with support of previous studies show there is a possibility that barley extract could be used as a more eco-friendly alternative to harmful chemicals to rid lakes and rivers of algae (Geiger et al. 2005). The barley extract does not kill algae it only suppresses the reproduction rate by working as an algistatic agent. The Environmental Protection Agency (EPA) states that barley straw is not considered a pesticide and is therefore not regulated (Lembi 2002). This is a better alternative than introducing a toxic chemical to kill the algae (Xi et al. 2010; Zhou 2010). Livestock and humans can more safely use bodies of water that do not have a bad taste or odor and swimming and fishing will increase with the reduction of algae in lakes (Ferrier et al. 2005).
Acknowledgements
I would like to acknowledge Dr. Deaver for her assistance on this project, Jim Carver for mixing my solutions, and Cara Carson and Tony Ross for help in the lab.
Literature Cited
American Public Health Association. 1989. Standard Methods for the Examination of Water and Wastewater, 17 th Edition, American Public Health Association Washington, DC. Pp. 10-31-10-34.
Ball, A.S., M. Williams, D. Vincent and J. Robinson. 2000. Algal growth control by a barley straw extract. Bioresource Technology 77: 177-181.
Bellinger, E. and D. Sigee. 2010. Freshwater Algae Identification and Use as Bioindicators. Wiley-Blackwell. Hoboken, NJ. Pp. 33-34, 153, 224-225, & 238.
Ferrier, M., B. Butler Sr., D. Terlizzi and R. Lacouture. 2005. The effects of barley straw ( Hordeum vulgare) on the growth of freshwater algae. Bioresource Technology 96: 1788-1795.
Geiger, S., E. Henry, P. Hayes, and K. Haggard. 2005. Barley straw-algae control literature analysis. South Dakota State University. http://www.sdstate.edu/ nrm/outreach/ pond/ upload/ barleyalgae-control.pdf. (Accessed 03/27/2012).
Graham, L., J. Graham, and L. Wilcox. 2009. Algae. Pearson Education, Inc., San Francisco,page 236.
Grover, J., J. Baker, F. Urena-Boeck, B. Brooks, R. Errera, D. Roelke, and R. Keisling. Laboratory tests of ammonium and barley straw extract as agents to suppress abundance of the harmful algae Prymnesium parvum and its toxicity to fish. Water Research 41: 2503-2512.
Hainz, R., C. Wöber and M. Schagerl. 2009. The relationship between Spirogyra(Zygnematophyceae, Streptophyta) filament type groups and environmental conditions in Central Europe. Aquatic Botany 91:173-180.
Hosja, W. 1998.Water Facts: Algal Bloom. Water and Rivers Commission 6:1-15.
James, D. 1978. Culturing Algae. Carolina Biological Supply Company. Burlington, North Carolina. page 12.
Kumar, H., S. Gajaria, S. and K. Radha. 2004. Growth and development of catla ( Catla catla) fed with different levels of diet containing Spirogyra sp. Bioresource Technology 95:74-76.
Lembi, C. 2002. Barley straw for algae control. Aquatic Plant Management. http://www.ces.purdue.edu/extmedia/ws/ws_21.pdf. (Accessed 03/27/2012).
Parsons, T., Y. Maita and C. Lalli, C. 1984. A manual of chemical and biological methods for seawater analysis. Pergamon Press, Inc. Maxwell, New York. Pp. 101-104.
Valkama, E., R. Uusitalo, K. Ylivainio, P. Virkajarvi and E. Turtola. 2009 Phosphorus fertilization: a meta-analysis of 80 years of research in Finland. Agriculture, Ecosystems and Environment 130: 75-85.
Xi, X., C. Ying-xu, L. Xin-qiang, L. Li-ping, and T. Xian-jin. 2010. Effects of Tibetan hulless barley on bloom-forming cyanobacterium ( Microcystis aeruginosa) measured by different physiological and morphologic parameters. Chemosphere 81:1118-1123.
Zhou, J. 2010. Inhibitory effect of decomposing barley on algal growth in water and wastewater. ISTC Reports RR-118 pp. 1-30.